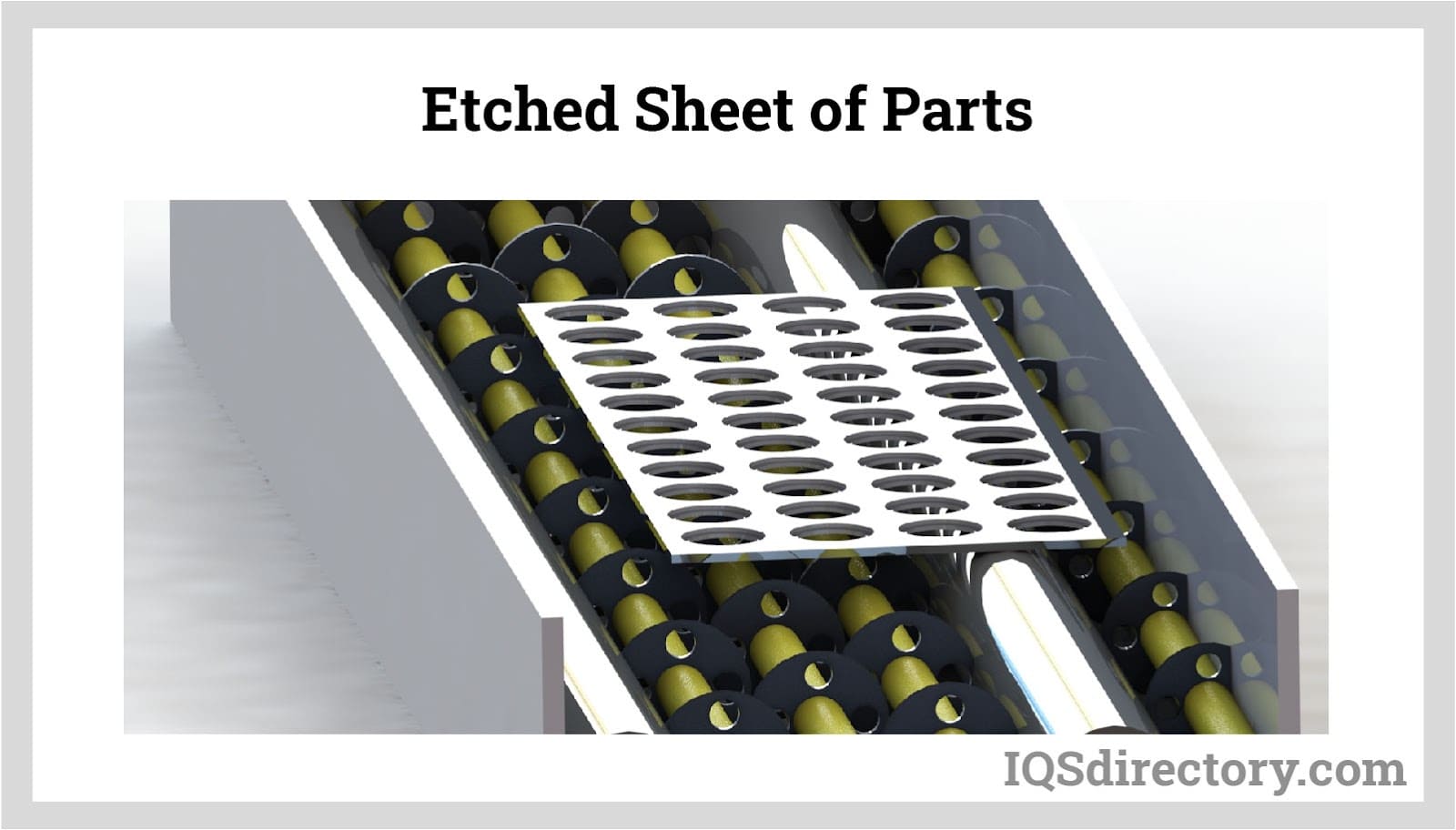
Etching
Much like the acid etching process, the developed sheets are placed on a conveyor that moves the sheets through a machine that pours etchant on the sheets. Where the etchant connects with the exposed metal, it dissolves the metal leaving the protected material.
In most photochemical processes, the etchant is ferric chloride, which is sprayed from the bottom and top of the conveyor. Ferric chloride is chosen as an etchant because it is safe to use and recyclable. Cupric chloride is used to etch copper and its alloys.
The etching process has to be carefully timed and is controlled in accordance with the metal that is being etched since some metals take longer to etch than others. For the success of photochemical etching, careful monitoring and control are crucial.
In the etching stage of photochemical metal etching, the developed metal sheets are placed on a conveyor that moves them through a machine where etchant is poured onto the sheets. The etchant dissolves the exposed metal, leaving behind the protected areas of the sheet.
Ferric chloride is commonly used as an etchant in most photochemical processes because it is safe to use and can be recycled. For copper and its alloys, cupric chloride is used instead.
The etching process must be carefully timed and controlled according to the type of metal being etched, as some metals require longer etching times than others. To ensure the success of the photochemical etching process, careful monitoring and control are crucial.